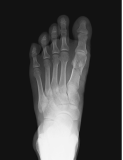
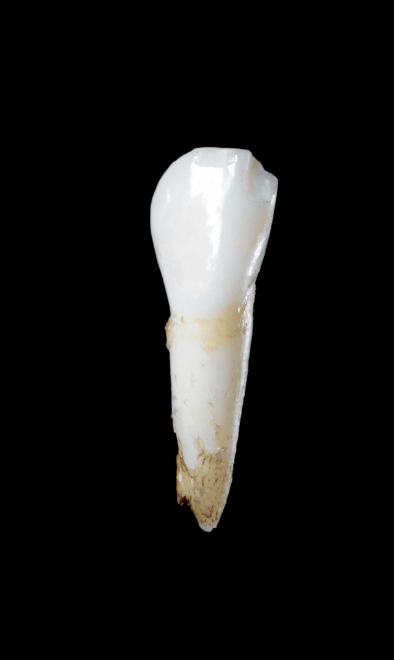
References: 1. Bishop N, Munns CF, Ozono K. Transformative
therapy in hypophosphatasia. Arch Dis Child. 2016;101(6):514-515.
2.
Desborough R, Nicklin P, Gossiel F, et al. Clinical and biochemical characteristics
of adults with hypophosphatasia attending a metabolic bone clinic. Bone. 2021;144:115795. 3. Bloch-Zupan A. Hypophosphatasia: diagnosis
and clinical signs - a dental surgeon perspective. Int J Paediatr Dent. 2016;26(6):426-438.
4. Shapiro JR, Lewiecki EM. Hypophosphatasia in adults: clinical
assessment and treatment considerations. J Bone Miner Res. 2017;32(10):1977-1980.
5. Rockman-Greenberg C. Hypophosphatasia. Pediatr Endocrinol Rev. 2013;10(suppl 2):380-388. 6. McKiernan FE, Berg RL, Fuehrer
J. Clinical and radiographic findings in adults with persistent hypophosphatasemia.
J Bone Miner Res. 2014;29(7):1651-1660. 7. Whyte
MP. Hypophosphatasia: nature's window on alkaline phosphatase function in humans.
In: Bilezikian JP, Raisz LG, Martin TJ, eds. Principles of Bone Biology. 3rd ed. Academic Press; 2008:1573-1598.
8. Bianchi ML, Bishop NJ, Guañabens N, et al; Rare Bone Disease
Action Group of the European Calcified Tissue Society. Hypophosphatasia in
adolescents and adults: overview of diagnosis and treatment. Osteoporos Int.
2020;31(8):1445-1460. 9. Vieira LHR, Peixoto KC, Flósi CL,
Fleiuss de Farias ML, Madeira M. Active search of adult patients with persistently
low serum alkaline phosphatase levels for diagnosis of hypophosphatasia. Arch Endocrinol Metab. 2021;65(3):289-294. 10. NORD. Hypophosphatasia. Accessed
March 30, 2023. https://rarediseases.org/rare-diseases/hypophosphatasia/
11.
Mornet E, Nunes ME. Hypophosphatasia. In: Adam MP, Ardinger HH, Pagon RA, et
al, eds. GeneReviews®. University of Washington; 2007. Accessed March
30, 2023.
https://www.ncbi.nlm.nih.gov/books/NBK1150/
12. Mornet E. Molecular genetics of hypophosphatasia and phenotype-genotype
correlations. Subcell Biochem. 2015;76:25-43. 13.
Kamińska J, Dymicka-Piekarska V, Tomaszewska J, Matowicka-Karna J, Koper-Lenkiewicz
OM. Diagnostic utility of protein in creatinine ratio (P/C ratio) in spot urine
sample within routine clinical practice. Crit Rev Clin Lab Sci. 2020;57(5):345-364.
14. Shajani-Yi Z, Ayala-Lopez N, Black M, Dahir KM. Urine
phosphoethanolamine is a specific biomarker for hypophosphatasia in adults.
Bone. 2022;163:116504. 15. Phillips KA, Deverka PA,
Hooker GW, Douglas MP. Genetic test availability and spending: where are we
now? Where are we going? Health Aff (Millwood). 2018;37(5):710-716.
16. Mornet E. Hypophosphatasia. Metabolism. 2018;82:142–155.
17. Khan AA, Josse R, Kannu P, et al. Hypophosphatasia: Canadian
update on diagnosis and management. Osteoporos Int. 2019;30(9):1713-1722.
18.
NIH MedlinePlus. ALPL gene. National Library of Medicine. 2018. Accessed March
30, 2023. https://medlineplus.gov/genetics/gene/alpl/
19.
Horton RH, Lucassen AM. Recent developments in genetic/genomic medicine. Clin Sci (Lond). 2019;133(5):697-708. 20. Weiss MJ, Cole DE, Ray K, et al.
A missense mutation in the human liver/bone/kidney alkaline phosphatase gene
causing a lethal form of hypophosphatasia. Proc Natl Acad Sci. 1988;85:7666-7669.
21. The ALPL Gene Variant Database. JKU Faculty of Medicine.
Accessed March 30, 2023.
https://alplmutationdatabase.jku.at/
22. Jandl NM, Schmidt T, Rolvien T, et al. Genotype-phenotype
associations in 72 adults with suspected ALPL-associated hypophosphatasia.
Calc Tissue Int. 2021;108(3):288-301. 23. Nunes ME.
Hypophosphatasia.
GeneReviews®. 2007;1993–2002. 24. Whyte MP, Leung
E, Wilcox WR, et al. Natural history of perinatal and infantile hypophosphatasia:
a retrospective study. J Pediatr. 2019;209:116-124.e4. 25. Zurutuza L, Muller F, Gibrat JF, et al. Correlations of genotype and phenotype
in hypophosphatasia.
Hum Mol Genet. 1999;8(6):1039-1046. 26. Hofmann C,
Girschick HJ, Mentrup B, et al. Clinical aspects of hypophosphatasia: an update.
Clin Rev Bone Miner Metab. 2013;11:60-70. 27. Sanabria-de
la Torre R, Martínez-Heredia L, González-Salvatierra S, et al. Characterization
of genetic variants of uncertain significance for the ALPL gene in patients
with adult hypophosphatasia. Front Endocrinol (Lausanne). 2022;13:863940.
28.
Blueprint Genetics. VUS—the most maligned result in genetic testing. 2019.
Accessed March 30, 2023.
https://blueprintgenetics.com/resources/vus-the-most-maligned-result-in-genetic-testing/
29. Beck NM, Sagaser KG, Lawson CS, et al. Not just a carrier:
clinical presentation and management of patients with heterozygous disease-causing
alkaline phosphatase (ALPL) variants identified through expanded carrier screening.
Mol Genet Genomic Med. 2023;11(1):e2056. 30. Kishnani
PS, Rockman-Greenberg C, Rauch F, et al. Five-year efficacy and safety of asfotase
alfa therapy for adults and adolescents with hypophosphatasia. Bone. 2019;121:149-162.