How hypophosphatasia CAN cause harm
Adults with hypophosphatasia may experience a full spectrum of symptoms. In addition to the typical skeletal effects, pain and muscular/metabolic manifestations are also common in adults with hypophosphatasia, regardless of age of onset.6,7*
*Adults may have a history of childhood symptoms.6
Studies of adult patients found:

Studies of adult patients found:
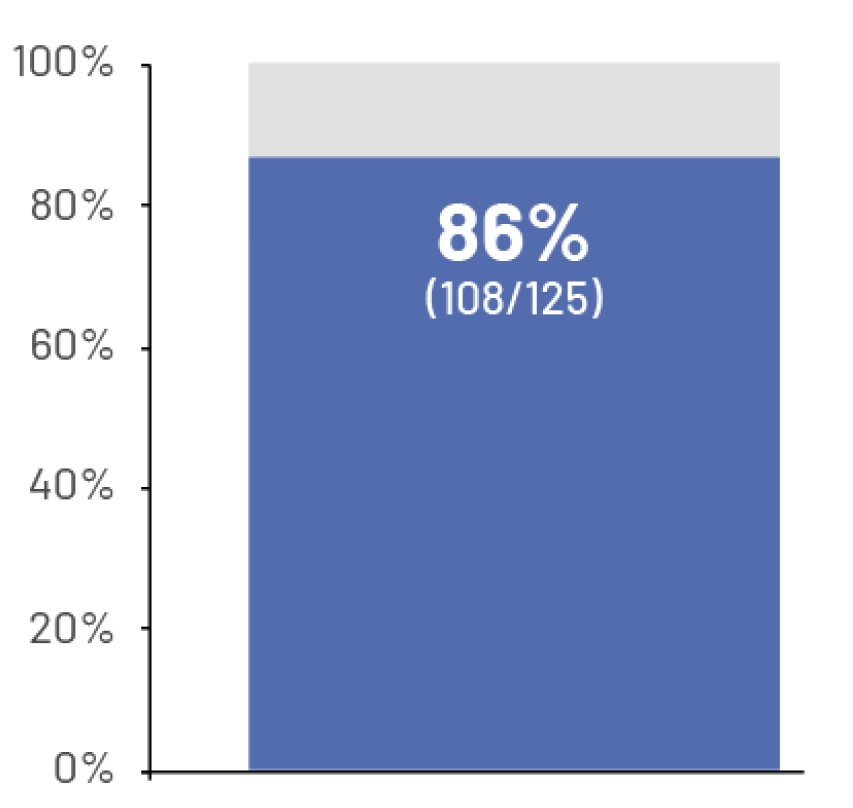
95%
of patients reported pain and 76% said bone pain was severe enough to limit activity7†
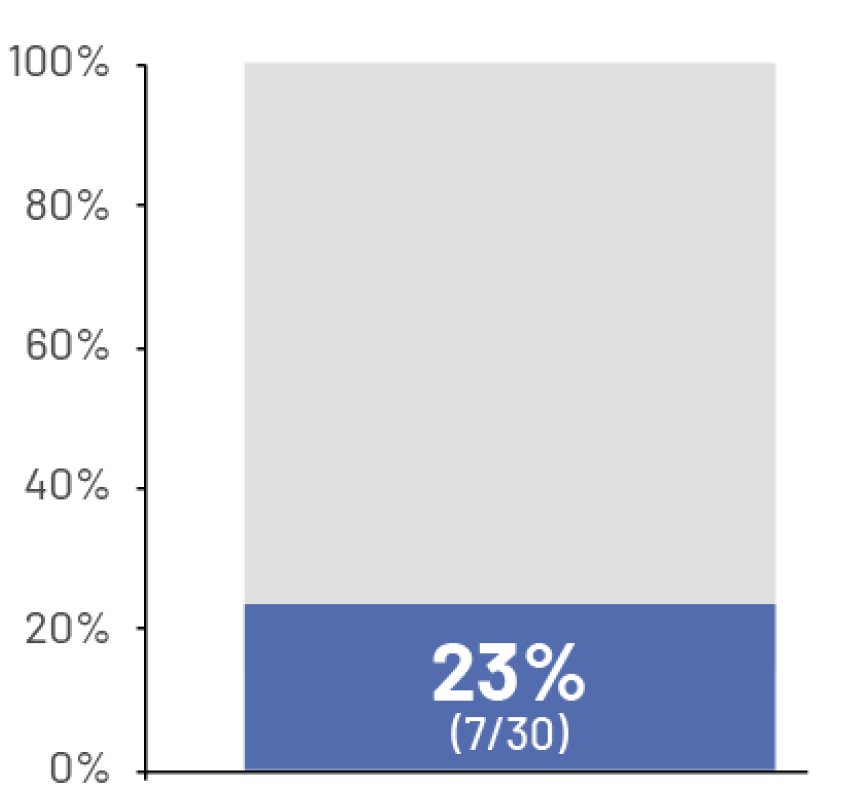
74%
of patients reported inability to climb stairs and 81% reported inability to descend stairs7†
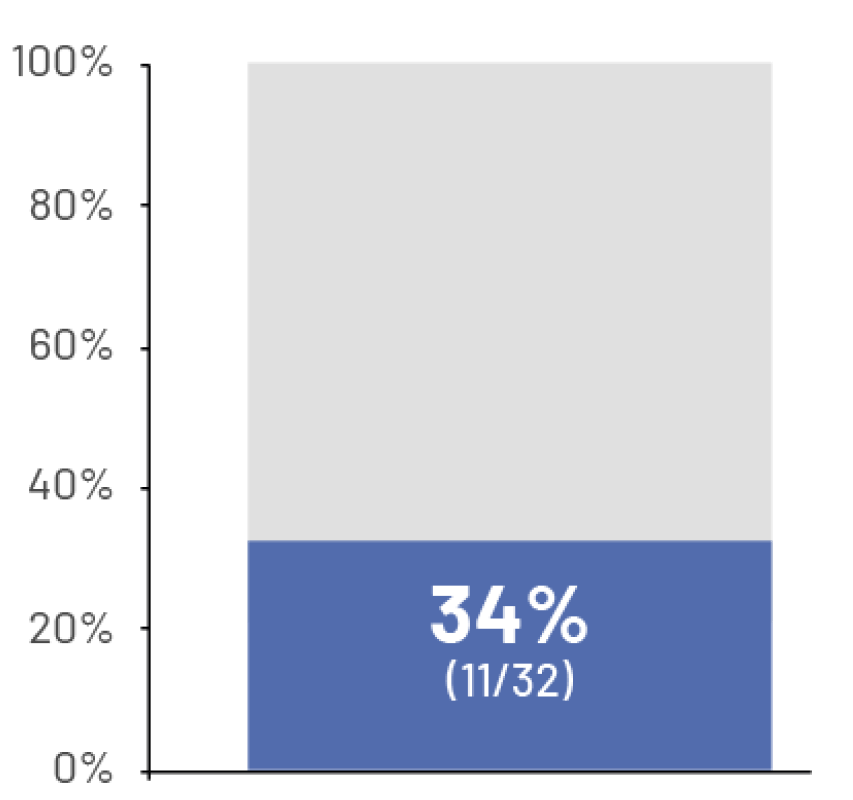
78%
had difficulty walking7†
>50%
A separate study of patients with hypophosphatasia reported their health problems negatively affected their physical and mental functioning8‡

†Data collected from patients and caregivers via 2 survey instruments (web-based and phone interviews) to evaluate patient-reported symptomatology and burden of disease of hypophosphatasia (N=125). The majority of patients in this study (67%) reported pediatric onset of hypophosphatasia symptoms. Of patients reporting adult-onset hypophosphatasia (27%), 50% (17/34) reported history or surgeries, suggestive of pediatric onset of symptoms.7
‡Data from the Global HPP Registry, an observational, longitudinal, multinational, long-term study of patients ≥18 years with a confirmed diagnosis of hypophosphatasia using physician-entered medical record data from consenting patients (N=304). Of the 304 adults, 33% (~100 patients) had pediatric-onset HPP.8
The multisystemic impact of hypophosphatasia1,6
Neurologic9
Fatigue, headaches, sleep disturbances, neuropathy, and hearing loss

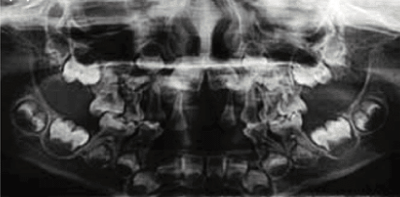
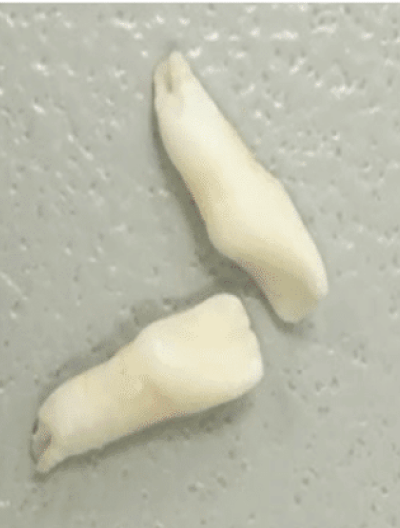
Dental1,10,11
Adult tooth loss, abnormal dentition, and periodontal disease

Disclaimer: This image is not taken from a hypophosphatasia (HPP) patient.
Muscular6,10,12
Muscle pain and weakness, muscular hypotonia, reduced grip force, history of abnormal gait, immobility, and tendon calcification

Reduced grip force assessed by digital hand dynamometer
Reproduced with permission from Jandl NM, et al. Calcif Tissue Int. 2021;108(3):288-301.
Rheumatic1,6,10
Calcific periarthritis, chondrocalcinosis, pain, pseudogout, and osteoarthropathy

Disclaimer: This image is not taken from a hypophosphatasia (HPP) patient.
Renal3,6,10

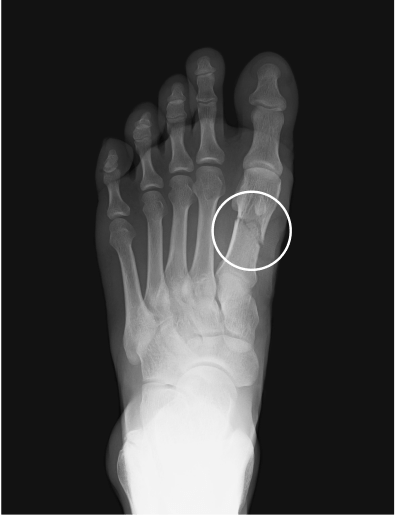
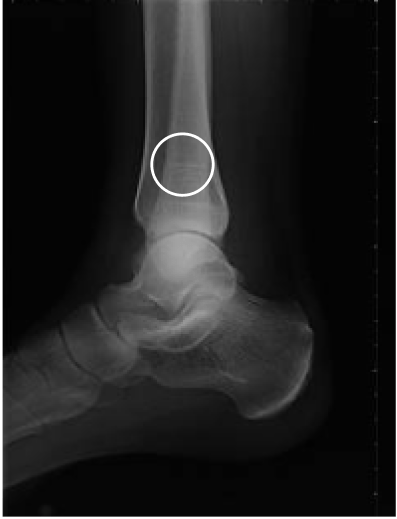
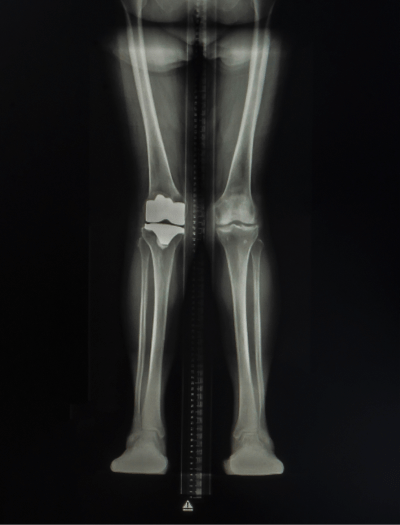
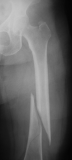
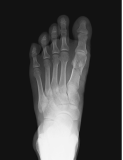
Orthopedic/skeletal1,3,6,10,13
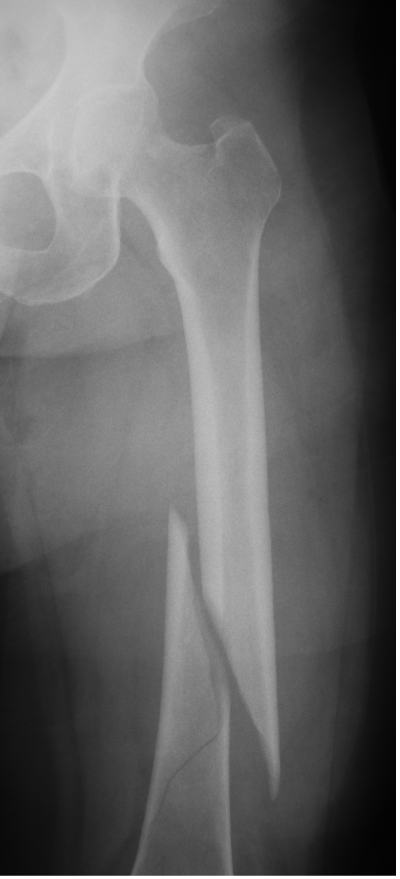
Fractures, pseudofractures, history of rickets-like changes, osteomalacia, hypomineralization, bone/joint pain, and history of bone deformities



Fatigue, headaches, sleep disturbances, neuropathy, and hearing loss



Adult tooth loss, abnormal dentition, and periodontal disease

Disclaimer: This image is not taken from a hypophosphatasia (HPP) patient.


Muscle pain and weakness, muscular hypotonia, reduced grip force, history of abnormal gait, immobility, and tendon calcification

Reduced grip force assessed by digital hand dynamometer
Reproduced with permission from Jandl NM, et al. Calcif Tissue Int. 2021;108(3):288-301.


Calcific periarthritis, chondrocalcinosis, pain, pseudogout, and osteoarthropathy

Disclaimer: This image is not taken from a hypophosphatasia (HPP) patient.





Fractures, pseudofractures, history of rickets-like changes, osteomalacia, hypomineralization, bone/joint pain, and history of bone deformities


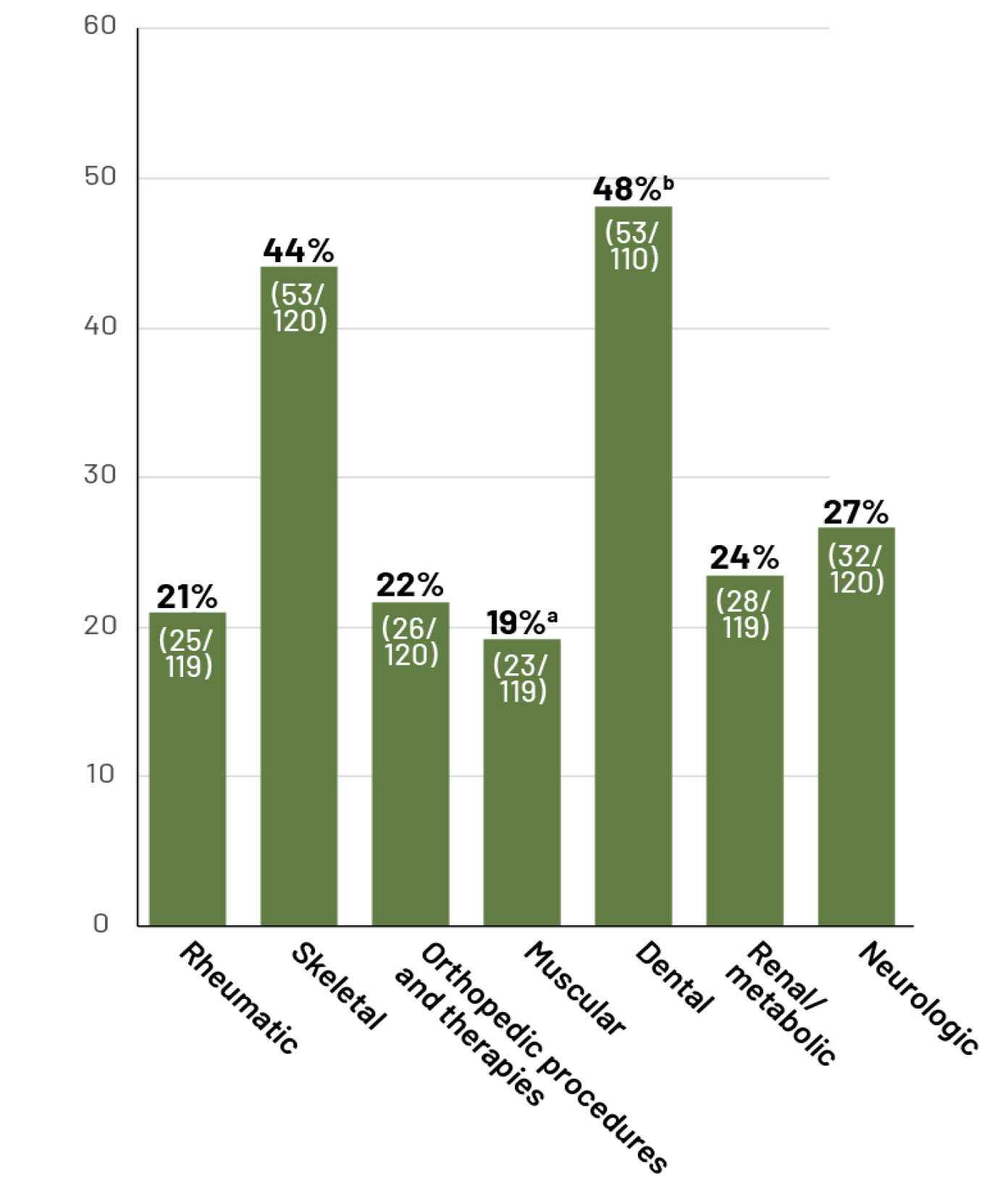
Selected reported body system signs/symptoms or interventions in adult patients with HPP6:
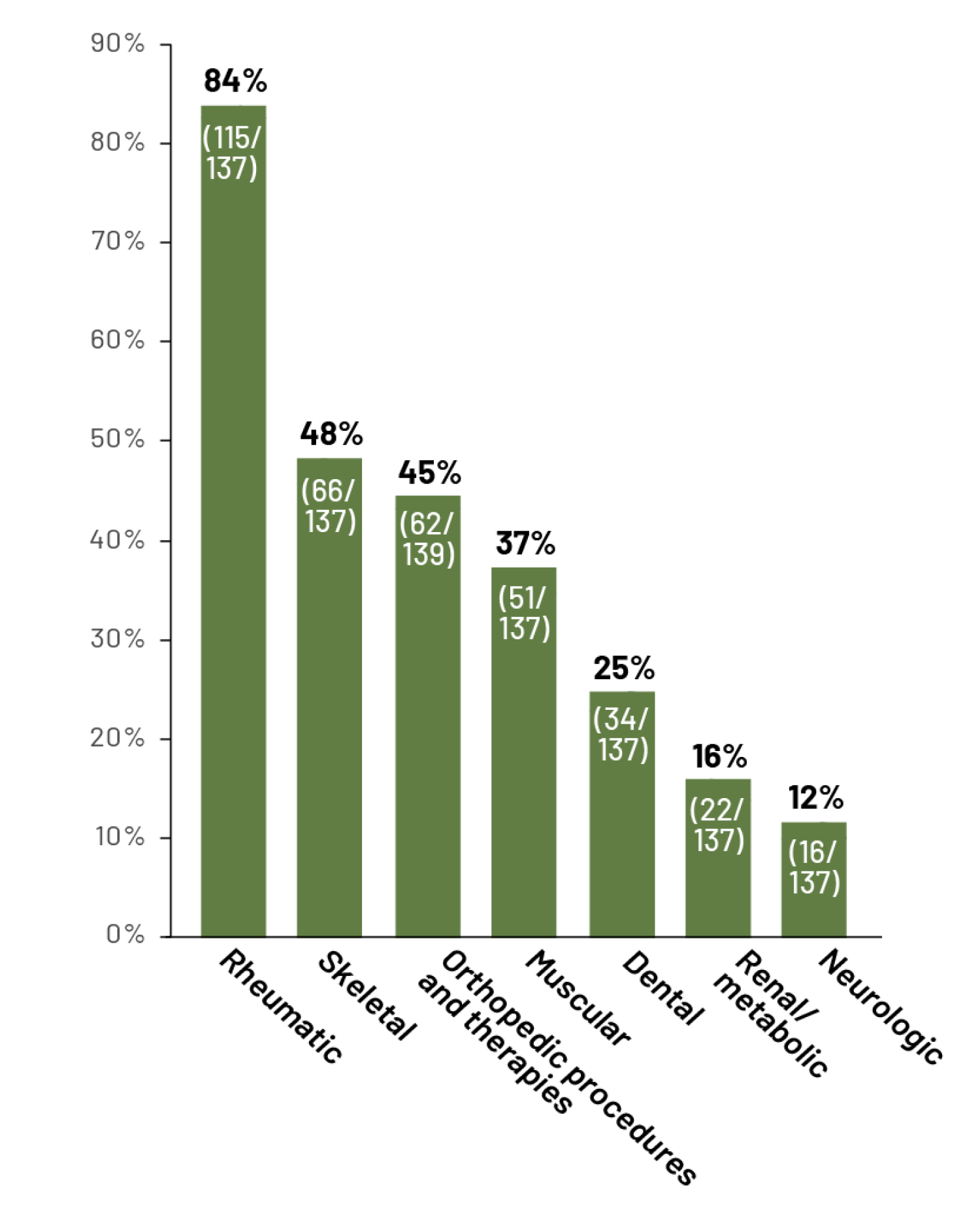
Observational, multinational, prospective Global HPP Registry study conducted in adults (n=148; ≥18 years) from January 2015 to September 2017.6
12%
of adult patients with hypophosphatasia have a history of neurologic symptoms6*
*Neurologic symptoms refer to craniosynostosis, developmental delay, increased intracranial pressure, and seizures.
A retrospective chart review studied the prevalence of common neurologic symptoms associated with hypophosphatasia9,a,b
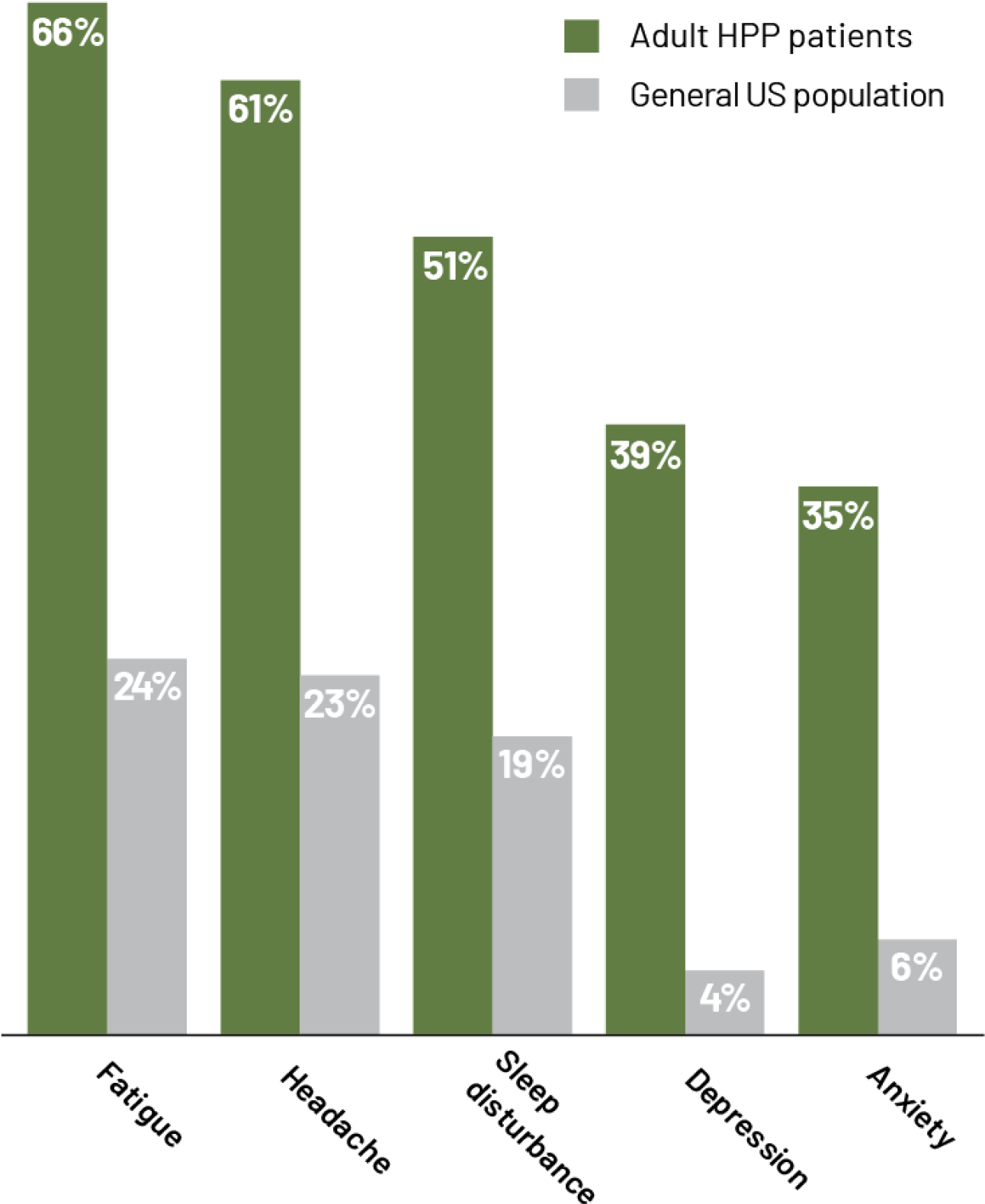
aPrevalence of neurologic symptoms in adults with hypophosphatasia (N=82) compared with the general US population.9
bData taken from a retrospective chart review performed on a series of 82 HPP patients.9
Hypophosphatasia CAN CAUSE INCREASING HARM
It’s vital to consider that current symptom presentation may not accurately represent the degree of disease progression. Patients with hypophosphatasia can experience a wide range of unexplained and debilitating symptoms that result in a frustrating journey to find relief. These clinical manifestations can change and accumulate, reflecting disease evolution and progression over time.1,6,8,9,14-16